I Am a Member of the Boron Family and Am the Most Abundant Metal in the Earth's Crust
The group 13 of the periodic table consists of the elements Boron, aluminium, gallium indium,thallium and the newly discovered elements ununtrium(uut).
Occurrence
Boron occurs in two forms ![]() .
.
Its affluence in the earth's crust is very depression. Boron mainly occur equally:
1) Orthoboric acid ,H3BO3
two)Borax, Na2[BivO5(OH)4].8H2O or Na2B4Oseven.10HiiO
three)kernite, Na2[BivOv(OH)4] or NaiiBivO7.2H2O
Aluminium is the third most abundant element by weight institute in the world crust afterward Oxygen and silicon.
The important minerals of aluminium are:
ane) Bauxite : AltwoO3.It may too be represented as AlO10(OH)3-2x.
ii)Cryolite (sodium aluminium fluoride) : NathreeAlFhalf-dozen
3)Mica(muscovite) :G2OAl2O3.6SiO2 or KAlSI3Oviii
four)Orthoclase (feldspar) : K2O.AltwoO3.6SiO2 or KAlSi3Oeight
5)Corundum( anhydrous alumina) : Al2O3
Gallium, indium and thallium are the less abundant than aluminium.
Electronic configuration
The general valence vanquish electronic configuration of elements of group 13 is nsi np1 where n=2-vii.
Boron and Aluminium have element of group 0 core, gallium and indium take noble gas +10 d-electrons and thallium has noble gas +14 f + 10d electrons core.
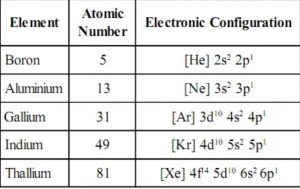
Atomic and Ionic radii
The atomic and ionic radii of group 13 elements are smaller than those of the respective elements of group 2.
Reason: On moving from left to right i.east. grouping 2 to group 13 ,the nuclear charge increases while the new electrons enter the same crush. Farther the electrons in the same vanquish exercise non screen each other. Therefore, the effective nuclear charge increases and outer electrons are pulled more strongly towards the nucleus. This results in decrease in atomic size.
On moving down the group, both atomic and ionic radii are expected to increase primarily due to addition of a new electron shell with each succeeding element. Notwithstanding, there are some deviations every bit nosotros movement from aluminium to gallium.
Reason : This is due to the filling of electrons in d orbital. In betwixt Al and Ga ,there are 10 elements of the outset transition series which have electrons in the inner d orbital. As the d orbitals are large in size, these intervening electrons exercise non shield the nucleus finer. Effective nuclear charge of Ga is greater in magnitude than that of Al. As a result, the electrons in Ga experience greater force of attraction by the nucleus than in Al and hence atomic radius of the Ga is slightly less than that of Al.
Ionisation enthalpy
The first ionization enthalpies of the elements of group 13 are lower than the corresponding elements of group 2.
Reason : Elements of group 13 accept 3 valence electrons in the valence shell; two of these are nowadays in the s- orbital and ane in the p-orbital. For the get-go ionisation enthalpy, the electron has to be removed from the p- orbitals in instance of group 13 elements whereas in case of alkaline earth metals the s-electron of the aforementioned principal shell has to be removed. Since an s-electron is nearer the nucleus, it is more than strongly attracted than the p-electron of the aforementioned master beat. Hence, the removal of p-electron is much easier than the s-electron and therefore, the first ionization enthalpies of the elements of grouping 13 are lower as compared to the corresponding values of the alkaline globe metals of grouping ii.
On moving downward the grouping 13 from Boron to aluminium, at that place is a precipitous decrease in kickoff ionization enthalpies of aluminium due to an increase in atomic size and screening event which outweigh the result of increased nuclear charge.
First ionisation enthalpy of gallium is just slightly higher than that of aluminium while that of Tl is much college than those of Al, Ga and In.
Reason: Al follows immediately after s-block elements while Ga and In follow after d-block elements and thallium after d and f block elements. These d and f electrons practice not shield the valence electrons from the nucleus very effectively than due south and p- electrons. As a result ,the valence electrons remain fairly tightly held past the nucleus and hence larger amount of energy is needed for their removal. Now as we move from Al to Ga , due to poor shielding of the nucleus past 3d electrons , the effective nuclear charge acting on gallium is slightly college than that of aluminium. As a result, starting time ionisation enthalpy of gallium is slightly higher than that of aluminium. On moving down the group from gallium although the nuclear charge increases past 18 units but the total shielding event of 3d and 4d electrons outweighs the effect of increased nuclear charge and hence first ionization enthalpy of In is lower than that of gallium.
On further moving down the group from indium to thallium the nuclear accuse increases considerably by 32 units which outweighs the shielding event of all the electrons of the inner shells including those of 4f and 5d electrons. As a effect, constructive nuclear accuse acting on thallium is much higher than that on In and hence beginning ionization enthalpy of thallium is much higher than that of In even higher than those of gallium and Aluminium.
The 2nd and third ionization enthalpies of these elements are quite higher than their corresponding first ionization enthalpies.
Electronegativity
The elements of boron family are more electronegative than the elements of alkali metals and alkaline earth metals. The electronegativity start decreases from B to Al and so increases down the group.
Reason: Equally we move from Boron to aluminium ,the atomic size increases ,considerably, therefore attraction of nucleus for the electron decreases and hence electronegativity decreases. Equally the size increases but the effective nuclear accuse increases due to poor shielding of the inner of the inner d and f electrons. As a result, force of attraction of the nucleus for the electrons increases and hence the electronegativity increases from Al to Tl.
Electropositive grapheme – metallic graphic symbol
The elements of group xiii are less electropositive or metallic as compared to alkali metal (group ane) and alkaline earth metal (group 2). On moving down the group, the electropositive character of the elements first increases from Boron to aluminium and then decreases from aluminium to thallium.
Reason: Amidst the elements of group xiii, Boron has the highest sum of showtime three ionization enthalpies i.due east.ΔiH1+ΔiH2 +ΔiH3.Every bit a result ,it has little tendency to lose electrons and hence is least electropositive among group 13 elements. It is a non-metallic and a poor conductor of electricity. As nosotros move from Boron to aluminium, the sum of ΔiH1+ΔiH2 +ΔiHthreedecreases due to increase in diminutive size and hence aluminium has a high tendency to lose electrons.Al is highly electropositive. Therefore ,Aluminium is a metal and a good conductor of electronegativity.
Since the electrode potential for the reaction, M3+ (aq) + 3e‾ ——-> M(due south) increases from aluminium to thallium, therefore, their electropositive character decreases i.e.Al(-1.66V) to Ga(-0.56V) to In(-0.34V) to Tl(1.26V).
Melting point and boiling signal
The melting point of group thirteen elements do not show a regular trend.This is probably due to unusual crystal structure of Boron and gallium. The melting point decreases sharply on moving downwards the grouping from B to Ga and then increase from Ga to Tl. Amongst the elements of group 13, gallium has the lowest melting point and could exist as a liquid at room temperature in summers.
Boron has high melting point because its crystal construction consists of icosahedral units with boron atoms at all the 12 corners and each Boron cantlet is bonded to 5 equidistant neighbours. The crystal structure of gallium is quite different from that of boron. Each gallium metal atom has 1 shut neighbour at a distance of 243pm and half dozen more than distant neighbours at a distance of and 279 pm270 pm. Ga consist of almost discrete diatomic molecule and hence its melting point is low.
Al, In and Tl have closed packed construction. Their melting point decreases from Al to In and increases again for Tl.
Density
Due to smaller atomic and ionic radii, the elements of group thirteen have higher densities equally compared to elements of group 2.
On moving down the group, the densities increases. This is due to increase in the atomic mass of the element which outweighs the effect of increased atomic size. The densities of boron and Aluminium are quite lower than those of other members.
Reader Interactions
Source: https://classnotes.org.in/class11/chemistry/p-block-elements/physical-properties-boron-family/
0 Response to "I Am a Member of the Boron Family and Am the Most Abundant Metal in the Earth's Crust"
Post a Comment